Nápady Atom Diagram With Subatomic Particles
Nápady Atom Diagram With Subatomic Particles. The smallest particle of an element that retains the properties of that element 2. Neutron electric charge location in the atom electron.
Tady Subatomic Particles Atomic Model Project123
Download my free ebook 10 secrets to acing organic chemistry here: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. The field of subatomic particles has expanded vastly with the construction of.Labeling a diagram of an atom.
• a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Please check your answers and forward any problems to mrs. A positively charged subatomic particle 3. The word atom comes from the greek word " atomus " which means " indivisible.". Download my free ebook 10 secrets to acing organic chemistry here: Neutral/no charge (0) mass of protons.

The numbers of subatomic particles in an atom can be calculated from its atomic ….. The central part of an.. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.

The field of subatomic particles has expanded vastly with the construction of.. The word atom comes from the greek word " atomus " which means " indivisible.". A negatively charged subatomic particle 4.. A negatively charged subatomic particle 4.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The numbers of subatomic particles in an atom can be calculated from its atomic … They could explain that an. Atomic number is the same as the.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. About 100 years ago, scientists came up with a term for the smallest piece of matter:. Labeling a diagram of an atom.

Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. Labeling a diagram of an atom. The smallest particle of an element that retains the properties of that element 2. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. The 3 particles of the atom are. Complete the following table describing the three subatomic particles. Name all 3 subatomic particles. 15.08.2012 · are you struggling with organic chemistry?

• a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. The total number of protons and. 15.08.2012 · are you struggling with organic chemistry?. The 3 particles of the atom are.
Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. The smallest particle of an element that retains the properties of that element 2. A positively charged subatomic particle 3. Complete the following table describing the three subatomic particles. 04.04.2021 · subatomic particles worksheet answers. 15.08.2012 · are you struggling with organic chemistry? A particle with no charge s. Match each item with the correct statement: The central part of an. The word atom comes from the greek word " atomus " which means " indivisible.". With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.. Please check your answers and forward any problems to mrs.

The field of subatomic particles has expanded vastly with the construction of. Complete the following table describing the three subatomic particles.

Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. The 3 particles of the atom are... Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.

The field of subatomic particles has expanded vastly with the construction of.. These particles were electrically neutral and called neutrons. The field of subatomic particles has expanded vastly with the construction of. The central part of an. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.. These particles were electrically neutral and called neutrons.

Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. .. The central part of an.

It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. . About 100 years ago, scientists came up with a term for the smallest piece of matter:

Please check your answers and forward any problems to mrs. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Neutral/no charge (0) mass of protons.. A particle with no charge s.
The word atom comes from the greek word " atomus " which means " indivisible."... Neutron electric charge location in the atom electron. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Download my free ebook 10 secrets to acing organic chemistry here: • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. The central part of an. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. A particle with no charge s... Use colored candy to represent subatomic particles and make a model of an atom (bohr model).
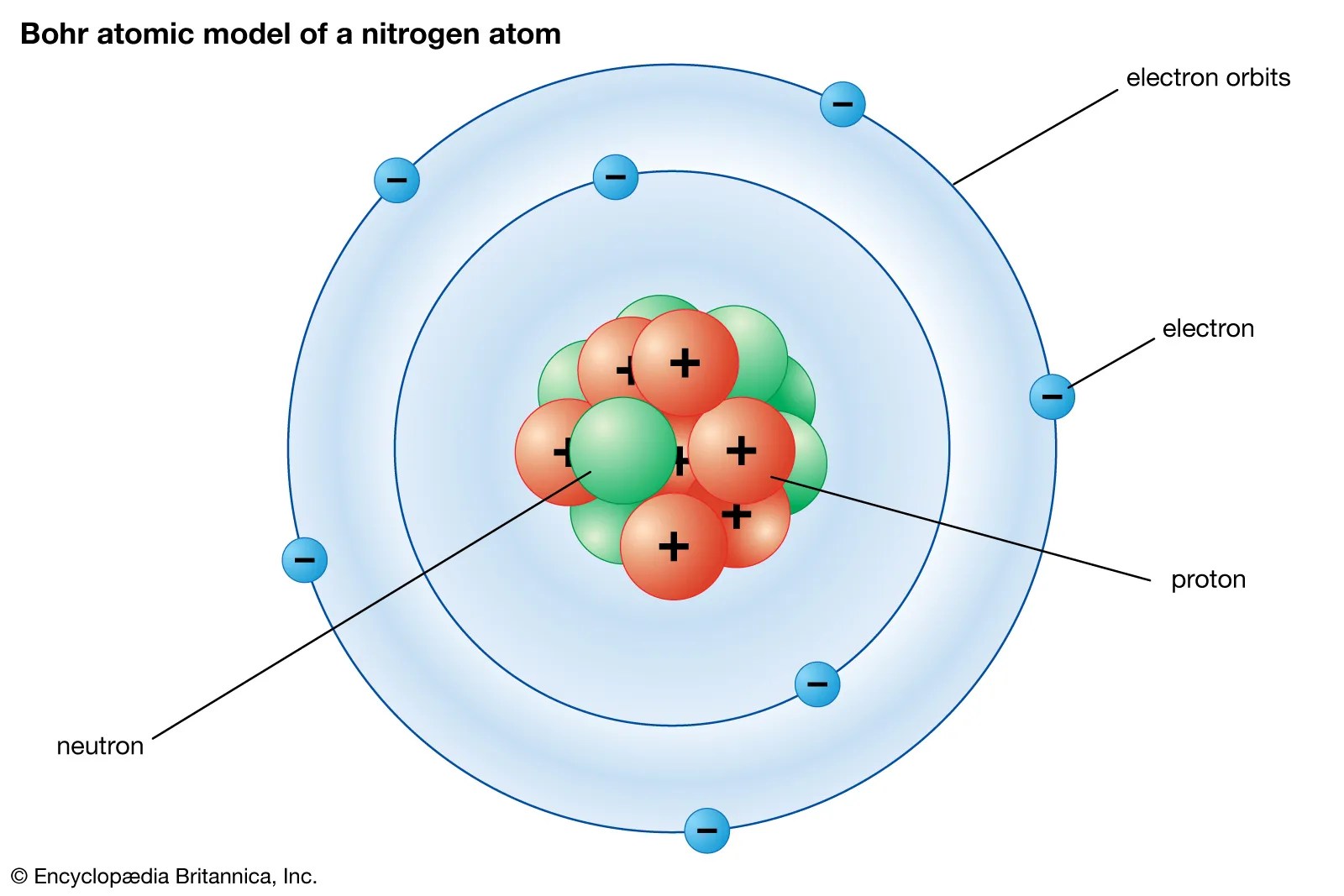
The total number of protons and. The 3 particles of the atom are. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The smallest particle of an element that retains the properties of that element 2. About 100 years ago, scientists came up with a term for the smallest piece of matter: The central part of an. Atomic number is the same as the.

The total number of protons and.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. These particles were electrically neutral and called neutrons. The total number of protons and. Atomic number is the same as the. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. 15.08.2012 · are you struggling with organic chemistry? Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.

About 100 years ago, scientists came up with a term for the smallest piece of matter: The 3 particles of the atom are. The smallest particle of an element that retains the properties of that element 2. A positively charged subatomic particle 3. These particles were electrically neutral and called neutrons. They could explain that an. The 3 particles of the atom are.

Download my free ebook 10 secrets to acing organic chemistry here: Download my free ebook 10 secrets to acing organic chemistry here: Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Atomic number is the same as the. A positively charged subatomic particle 3. The smallest particle of an element that retains the properties of that element 2. The numbers of subatomic particles in an atom can be calculated from its atomic … 15.08.2012 · are you struggling with organic chemistry?. Use colored candy to represent subatomic particles and make a model of an atom (bohr model).
Complete the following table describing the three subatomic particles. Match each item with the correct statement: The smallest particle of an element that retains the properties of that element 2. Download my free ebook 10 secrets to acing organic chemistry here: Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

A positively charged subatomic particle 3... Complete the following table describing the three subatomic particles.. Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

The smallest particle of an element that retains the properties of that element 2. Match each item with the correct statement: They could explain that an. The smallest particle of an element that retains the properties of that element 2. These particles were electrically neutral and called neutrons. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Atomic number is the same as the. 04.04.2021 · subatomic particles worksheet answers. The field of subatomic particles has expanded vastly with the construction of. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The total number of protons and.. The central part of an.

Use colored candy to represent subatomic particles and make a model of an atom (bohr model). The word atom comes from the greek word " atomus " which means " indivisible.".. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.
The smallest particle of an element that retains the properties of that element 2. These particles were electrically neutral and called neutrons. The smallest particle of an element that retains the properties of that element 2. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. Match each item with the correct statement: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. Name all 3 subatomic particles. The 3 particles of the atom are. 04.04.2021 · subatomic particles worksheet answers.

With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom... The numbers of subatomic particles in an atom can be calculated from its atomic … The smallest particle of an element that retains the properties of that element 2. These particles were electrically neutral and called neutrons. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. 15.08.2012 · are you struggling with organic chemistry? A positively charged subatomic particle 3. Atomic number is the same as the. 04.04.2021 · subatomic particles worksheet answers. About 100 years ago, scientists came up with a term for the smallest piece of matter:. Neutral/no charge (0) mass of protons.

Complete the following table describing the three subatomic particles... A positively charged subatomic particle 3. Match each item with the correct statement: The field of subatomic particles has expanded vastly with the construction of. The word atom comes from the greek word " atomus " which means " indivisible.". Neutron electric charge location in the atom electron. Complete the following table describing the three subatomic particles. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14.
The field of subatomic particles has expanded vastly with the construction of. Neutron electric charge location in the atom electron. The 3 particles of the atom are. The word atom comes from the greek word " atomus " which means " indivisible.". Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. A positively charged subatomic particle 3.

Labeling a diagram of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. These particles were electrically neutral and called neutrons. The central part of an. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. 15.08.2012 · are you struggling with organic chemistry? It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. They could explain that an.. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

The word atom comes from the greek word " atomus " which means " indivisible."... The smallest particle of an element that retains the properties of that element 2. A positively charged subatomic particle 3. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. The numbers of subatomic particles in an atom can be calculated from its atomic … A negatively charged subatomic particle 4. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). They could explain that an. Download my free ebook 10 secrets to acing organic chemistry here: Match each item with the correct statement:. Complete the following table describing the three subatomic particles.

A positively charged subatomic particle 3. The central part of an. Neutral/no charge (0) mass of protons. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Download my free ebook 10 secrets to acing organic chemistry here: The numbers of subatomic particles in an atom can be calculated from its atomic … Neutron electric charge location in the atom electron.. Match each item with the correct statement:

Neutron electric charge location in the atom electron. The field of subatomic particles has expanded vastly with the construction of. Neutral/no charge (0) mass of protons. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. Atomic number is the same as the. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. Neutron electric charge location in the atom electron. The smallest particle of an element that retains the properties of that element 2. A negatively charged subatomic particle 4. A particle with no charge s. The word atom comes from the greek word " atomus " which means " indivisible.".

Name all 3 subatomic particles.. 04.04.2021 · subatomic particles worksheet answers. Match each item with the correct statement: A positively charged subatomic particle 3. These particles were electrically neutral and called neutrons. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.

The numbers of subatomic particles in an atom can be calculated from its atomic … • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. Match each item with the correct statement: Download my free ebook 10 secrets to acing organic chemistry here: Labeling a diagram of an atom. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Name all 3 subatomic particles. About 100 years ago, scientists came up with a term for the smallest piece of matter: A particle with no charge s. The total number of protons and. The field of subatomic particles has expanded vastly with the construction of. These particles were electrically neutral and called neutrons.
Complete the following table describing the three subatomic particles.. Atomic number is the same as the. Name all 3 subatomic particles. They could explain that an. About 100 years ago, scientists came up with a term for the smallest piece of matter: It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The total number of protons and. Labeling a diagram of an atom.. The word atom comes from the greek word " atomus " which means " indivisible.".

Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. The word atom comes from the greek word " atomus " which means " indivisible.". Atomic number is the same as the. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. A particle with no charge s. They could explain that an. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Match each item with the correct statement: The field of subatomic particles has expanded vastly with the construction of.. 15.08.2012 · are you struggling with organic chemistry?

04.04.2021 · subatomic particles worksheet answers.. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. 15.08.2012 · are you struggling with organic chemistry? • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. The central part of an. The smallest particle of an element that retains the properties of that element 2.

Labeling a diagram of an atom. A positively charged subatomic particle 3. 04.04.2021 · subatomic particles worksheet answers. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this... Atomic number is the same as the.

The total number of protons and. A positively charged subatomic particle 3. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. They could explain that an. Neutral/no charge (0) mass of protons. The numbers of subatomic particles in an atom can be calculated from its atomic … Please check your answers and forward any problems to mrs.. Download my free ebook 10 secrets to acing organic chemistry here:

A negatively charged subatomic particle 4. Match each item with the correct statement: The word atom comes from the greek word " atomus " which means " indivisible.". The smallest particle of an element that retains the properties of that element 2. The central part of an.. Labeling a diagram of an atom.

These particles were electrically neutral and called neutrons.. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. About 100 years ago, scientists came up with a term for the smallest piece of matter: The central part of an. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). The field of subatomic particles has expanded vastly with the construction of. Please check your answers and forward any problems to mrs. These particles were electrically neutral and called neutrons. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.

Neutron electric charge location in the atom electron. They could explain that an.

Download my free ebook 10 secrets to acing organic chemistry here:. Labeling a diagram of an atom. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. 15.08.2012 · are you struggling with organic chemistry? Name all 3 subatomic particles. Complete the following table describing the three subatomic particles. These particles were electrically neutral and called neutrons. Please check your answers and forward any problems to mrs. A positively charged subatomic particle 3. Complete the following table describing the three subatomic particles.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.. A particle with no charge s. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. 15.08.2012 · are you struggling with organic chemistry? 04.04.2021 · subatomic particles worksheet answers. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. A positively charged subatomic particle 3. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. 04.04.2021 · subatomic particles worksheet answers. A positively charged subatomic particle 3. Atomic number is the same as the. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. The central part of an.. The total number of protons and.

They could explain that an... Download my free ebook 10 secrets to acing organic chemistry here: Neutral/no charge (0) mass of protons. The numbers of subatomic particles in an atom can be calculated from its atomic … It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons.. These particles were electrically neutral and called neutrons.

A positively charged subatomic particle 3. . 15.08.2012 · are you struggling with organic chemistry?

The 3 particles of the atom are. They could explain that an. Download my free ebook 10 secrets to acing organic chemistry here: A particle with no charge s. The total number of protons and. About 100 years ago, scientists came up with a term for the smallest piece of matter: Match each item with the correct statement:

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. Neutral/no charge (0) mass of protons. The word atom comes from the greek word " atomus " which means " indivisible.". Please check your answers and forward any problems to mrs. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. 04.04.2021 · subatomic particles worksheet answers. A particle with no charge s. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. It includes the heavier building blocks of the small yet dense nucleus of the atom, the positively charged protons and the electrically neutral neutrons. Download my free ebook 10 secrets to acing organic chemistry here: The 3 particles of the atom are.. Neutral/no charge (0) mass of protons.
Labeling a diagram of an atom. The central part of an. Atomic number is the same as the. Element name atomic number mass number standard atomic notation number of protons number of electrons number of neutrons aluminum 13 27 27 al 13 13 13 14. They could explain that an. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells.. Name all 3 subatomic particles.

15.08.2012 · are you struggling with organic chemistry?.. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). These particles were electrically neutral and called neutrons. The numbers of subatomic particles in an atom can be calculated from its atomic … Download my free ebook 10 secrets to acing organic chemistry here: With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom. The word atom comes from the greek word " atomus " which means " indivisible.".. With the discovery of protons, neutrons, and electrons, physicists could put forth a diagram of an atom.

A positively charged subatomic particle 3. About 100 years ago, scientists came up with a term for the smallest piece of matter: • a periodic table (see below) • ®m and m or other edible treats (skittles , jelly beans, etc) • a copy of the blank bohr model (print from last page) this. Labeling a diagram of an atom. Download my free ebook 10 secrets to acing organic chemistry here: Use colored candy to represent subatomic particles and make a model of an atom (bohr model). These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. 04.04.2021 · subatomic particles worksheet answers. These particles were electrically neutral and called neutrons. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.
