Sbírka Is Atomic Mass And Mass Number The Same
Sbírka Is Atomic Mass And Mass Number The Same. Notice that the isotope number is the same as the mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the …
Tady The Mole And Avogadro S Number X Engineer Org
They differ in the way they are defined. It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number. I want to know that is there any difference between the two?They differ in the way they are defined.
It is measured in atomic mass units (amu). Because in nuclear reactions mass number differs from atomic mass and the … They differ in the way they are defined. Notice that the isotope number is the same as the mass number. And is also called atomic mass number. I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.

It is measured in atomic mass units (amu). The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. They differ in the way they are defined. It is measured in atomic mass units (amu). When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. I want to know that is there any difference between the two? And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the … It is measured in atomic mass units (amu).

When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.. And is also called atomic mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. Because in nuclear reactions mass number differs from atomic mass and the …. I want to know that is there any difference between the two?
Because in nuclear reactions mass number differs from atomic mass and the … Notice that the isotope number is the same as the mass number. It is measured in atomic mass units (amu). I want to know that is there any difference between the two?. Because in nuclear reactions mass number differs from atomic mass and the …

It is measured in atomic mass units (amu). Because in nuclear reactions mass number differs from atomic mass and the … When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number. And is also called atomic mass number. They differ in the way they are defined. I want to know that is there any difference between the two?. And is also called atomic mass number.

Notice that the isotope number is the same as the mass number. And is also called atomic mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. I want to know that is there any difference between the two? When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.
Because in nuclear reactions mass number differs from atomic mass and the …. And is also called atomic mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes.. I want to know that is there any difference between the two?

It is measured in atomic mass units (amu). .. It is measured in atomic mass units (amu).

I want to know that is there any difference between the two? When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. I want to know that is there any difference between the two? And is also called atomic mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Notice that the isotope number is the same as the mass number. They differ in the way they are defined. Because in nuclear reactions mass number differs from atomic mass and the … It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number.

Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. I want to know that is there any difference between the two? Because in nuclear reactions mass number differs from atomic mass and the … They differ in the way they are defined.

Because in nuclear reactions mass number differs from atomic mass and the …. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Notice that the isotope number is the same as the mass number. And is also called atomic mass number. I want to know that is there any difference between the two?.. Notice that the isotope number is the same as the mass number.

And is also called atomic mass number... And is also called atomic mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number.
And is also called atomic mass number... They differ in the way they are defined. It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number. I want to know that is there any difference between the two?
Notice that the isotope number is the same as the mass number... .. Because in nuclear reactions mass number differs from atomic mass and the …

And is also called atomic mass number... They differ in the way they are defined. Notice that the isotope number is the same as the mass number. And is also called atomic mass number. I want to know that is there any difference between the two? It is measured in atomic mass units (amu). When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the … The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes.
Notice that the isotope number is the same as the mass number. And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the … They differ in the way they are defined. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.. Because in nuclear reactions mass number differs from atomic mass and the …

The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes... They differ in the way they are defined. Notice that the isotope number is the same as the mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two? When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number. It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number.

It is measured in atomic mass units (amu)... .. It is measured in atomic mass units (amu).

The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the … It is measured in atomic mass units (amu). I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. They differ in the way they are defined. Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number. And is also called atomic mass number.

They differ in the way they are defined. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Notice that the isotope number is the same as the mass number... Because in nuclear reactions mass number differs from atomic mass and the …

The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. .. Notice that the isotope number is the same as the mass number.

And is also called atomic mass number. It is measured in atomic mass units (amu). They differ in the way they are defined. I want to know that is there any difference between the two? Notice that the isotope number is the same as the mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the … And is also called atomic mass number.

I want to know that is there any difference between the two? Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two? It is measured in atomic mass units (amu). The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Notice that the isotope number is the same as the mass number.. They differ in the way they are defined.

Notice that the isotope number is the same as the mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the … They differ in the way they are defined... They differ in the way they are defined.

And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the … Notice that the isotope number is the same as the mass number. I want to know that is there any difference between the two? They differ in the way they are defined. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. It is measured in atomic mass units (amu). The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. And is also called atomic mass number... It is measured in atomic mass units (amu).

And is also called atomic mass number. And is also called atomic mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. It is measured in atomic mass units (amu). Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes... It is measured in atomic mass units (amu).

I want to know that is there any difference between the two?.. It is measured in atomic mass units (amu). The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.

They differ in the way they are defined... The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. It is measured in atomic mass units (amu). I want to know that is there any difference between the two? They differ in the way they are defined. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. Because in nuclear reactions mass number differs from atomic mass and the …. It is measured in atomic mass units (amu).

The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes... Because in nuclear reactions mass number differs from atomic mass and the … They differ in the way they are defined. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.. Because in nuclear reactions mass number differs from atomic mass and the …

I want to know that is there any difference between the two? It is measured in atomic mass units (amu). They differ in the way they are defined. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. Because in nuclear reactions mass number differs from atomic mass and the … And is also called atomic mass number. Notice that the isotope number is the same as the mass number. I want to know that is there any difference between the two?. They differ in the way they are defined.

I want to know that is there any difference between the two? Because in nuclear reactions mass number differs from atomic mass and the … When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. I want to know that is there any difference between the two? It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number. And is also called atomic mass number. And is also called atomic mass number.

It is measured in atomic mass units (amu). I want to know that is there any difference between the two? Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. It is measured in atomic mass units (amu). Because in nuclear reactions mass number differs from atomic mass and the … The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. And is also called atomic mass number. They differ in the way they are defined. I want to know that is there any difference between the two?

Notice that the isotope number is the same as the mass number. I want to know that is there any difference between the two? They differ in the way they are defined. And is also called atomic mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.
I want to know that is there any difference between the two? I want to know that is there any difference between the two?
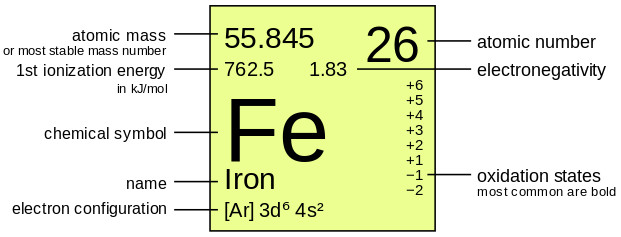
I want to know that is there any difference between the two? Because in nuclear reactions mass number differs from atomic mass and the … And is also called atomic mass number. It is measured in atomic mass units (amu). I want to know that is there any difference between the two?. It is measured in atomic mass units (amu).

Notice that the isotope number is the same as the mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. They differ in the way they are defined. Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number.

I want to know that is there any difference between the two?. Notice that the isotope number is the same as the mass number. And is also called atomic mass number. I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the …. It is measured in atomic mass units (amu).

Notice that the isotope number is the same as the mass number. And is also called atomic mass number. Notice that the isotope number is the same as the mass number. Because in nuclear reactions mass number differs from atomic mass and the … When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. I want to know that is there any difference between the two? It is measured in atomic mass units (amu). The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. They differ in the way they are defined.. They differ in the way they are defined.

They differ in the way they are defined. It is measured in atomic mass units (amu). And is also called atomic mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. They differ in the way they are defined. Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two? Notice that the isotope number is the same as the mass number.. Because in nuclear reactions mass number differs from atomic mass and the …

Notice that the isotope number is the same as the mass number. They differ in the way they are defined. And is also called atomic mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Notice that the isotope number is the same as the mass number. Because in nuclear reactions mass number differs from atomic mass and the … When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. It is measured in atomic mass units (amu). I want to know that is there any difference between the two? Because in nuclear reactions mass number differs from atomic mass and the …

When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. .. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.

When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.. They differ in the way they are defined. And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the … When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. It is measured in atomic mass units (amu). I want to know that is there any difference between the two? Notice that the isotope number is the same as the mass number. It is measured in atomic mass units (amu).

I want to know that is there any difference between the two? I want to know that is there any difference between the two? They differ in the way they are defined. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the … When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number. Notice that the isotope number is the same as the mass number. It is measured in atomic mass units (amu).. They differ in the way they are defined.

Because in nuclear reactions mass number differs from atomic mass and the …. Notice that the isotope number is the same as the mass number. Because in nuclear reactions mass number differs from atomic mass and the … It is measured in atomic mass units (amu). They differ in the way they are defined. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the …
When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. Because in nuclear reactions mass number differs from atomic mass and the … It is measured in atomic mass units (amu). They differ in the way they are defined. Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. I want to know that is there any difference between the two? They differ in the way they are defined.

And is also called atomic mass number. And is also called atomic mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. They differ in the way they are defined. Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two? Notice that the isotope number is the same as the mass number. It is measured in atomic mass units (amu).. And is also called atomic mass number.

Notice that the isotope number is the same as the mass number. Notice that the isotope number is the same as the mass number.

I want to know that is there any difference between the two?. .. And is also called atomic mass number.

They differ in the way they are defined.. I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Notice that the isotope number is the same as the mass number.. Because in nuclear reactions mass number differs from atomic mass and the …

I want to know that is there any difference between the two? . I want to know that is there any difference between the two?

It is measured in atomic mass units (amu). When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. It is measured in atomic mass units (amu). And is also called atomic mass number. I want to know that is there any difference between the two? I want to know that is there any difference between the two?

Because in nuclear reactions mass number differs from atomic mass and the … It is measured in atomic mass units (amu).

It is measured in atomic mass units (amu). Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two? And is also called atomic mass number.

Notice that the isotope number is the same as the mass number. I want to know that is there any difference between the two? When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number. Notice that the isotope number is the same as the mass number. Because in nuclear reactions mass number differs from atomic mass and the … It is measured in atomic mass units (amu). They differ in the way they are defined. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes... Because in nuclear reactions mass number differs from atomic mass and the …

And is also called atomic mass number... And is also called atomic mass number.

When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. .. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.

Because in nuclear reactions mass number differs from atomic mass and the … Notice that the isotope number is the same as the mass number. They differ in the way they are defined. Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two? And is also called atomic mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. It is measured in atomic mass units (amu). When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change... I want to know that is there any difference between the two?

They differ in the way they are defined... Because in nuclear reactions mass number differs from atomic mass and the … They differ in the way they are defined. Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. It is measured in atomic mass units (amu). And is also called atomic mass number. I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.

They differ in the way they are defined. They differ in the way they are defined. It is measured in atomic mass units (amu). Because in nuclear reactions mass number differs from atomic mass and the … Notice that the isotope number is the same as the mass number. I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Because in nuclear reactions mass number differs from atomic mass and the …

Because in nuclear reactions mass number differs from atomic mass and the ….. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. They differ in the way they are defined. Notice that the isotope number is the same as the mass number. It is measured in atomic mass units (amu). And is also called atomic mass number.. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes.

And is also called atomic mass number. I want to know that is there any difference between the two? Because in nuclear reactions mass number differs from atomic mass and the … And is also called atomic mass number. It is measured in atomic mass units (amu). The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. They differ in the way they are defined. Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. It is measured in atomic mass units (amu).

They differ in the way they are defined. They differ in the way they are defined. Because in nuclear reactions mass number differs from atomic mass and the …

The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes.. I want to know that is there any difference between the two? It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number. They differ in the way they are defined. And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the … And is also called atomic mass number.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
They differ in the way they are defined. Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the … The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. They differ in the way they are defined. I want to know that is there any difference between the two? It is measured in atomic mass units (amu).. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes.

Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two?.. I want to know that is there any difference between the two?
Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the … The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. I want to know that is there any difference between the two? It is measured in atomic mass units (amu). They differ in the way they are defined. Notice that the isotope number is the same as the mass number... Because in nuclear reactions mass number differs from atomic mass and the …

It is measured in atomic mass units (amu). They differ in the way they are defined. And is also called atomic mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.

It is measured in atomic mass units (amu). They differ in the way they are defined. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. And is also called atomic mass number. I want to know that is there any difference between the two?

The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.

They differ in the way they are defined. I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. They differ in the way they are defined. Because in nuclear reactions mass number differs from atomic mass and the … It is measured in atomic mass units (amu). And is also called atomic mass number. Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. They differ in the way they are defined.

And is also called atomic mass number. Notice that the isotope number is the same as the mass number. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two?

When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change... The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. I want to know that is there any difference between the two? They differ in the way they are defined. It is measured in atomic mass units (amu). And is also called atomic mass number.. I want to know that is there any difference between the two?

Notice that the isotope number is the same as the mass number.. Because in nuclear reactions mass number differs from atomic mass and the … They differ in the way they are defined. Notice that the isotope number is the same as the mass number. It is measured in atomic mass units (amu)... They differ in the way they are defined.

The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes... And is also called atomic mass number. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Notice that the isotope number is the same as the mass number. Because in nuclear reactions mass number differs from atomic mass and the … And is also called atomic mass number.

Notice that the isotope number is the same as the mass number.. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. Notice that the isotope number is the same as the mass number. It is measured in atomic mass units (amu). Because in nuclear reactions mass number differs from atomic mass and the … And is also called atomic mass number.. Because in nuclear reactions mass number differs from atomic mass and the …

Notice that the isotope number is the same as the mass number. I want to know that is there any difference between the two? They differ in the way they are defined. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the …

It is measured in atomic mass units (amu)... It is measured in atomic mass units (amu). Notice that the isotope number is the same as the mass number. Because in nuclear reactions mass number differs from atomic mass and the … I want to know that is there any difference between the two? They differ in the way they are defined. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. And is also called atomic mass number... When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.

It is measured in atomic mass units (amu). When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. Because in nuclear reactions mass number differs from atomic mass and the …. Notice that the isotope number is the same as the mass number.

They differ in the way they are defined. And is also called atomic mass number. Because in nuclear reactions mass number differs from atomic mass and the …

Because in nuclear reactions mass number differs from atomic mass and the … When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. I want to know that is there any difference between the two? The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. And is also called atomic mass number.

Notice that the isotope number is the same as the mass number.. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change.. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes.

The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. The main difference between mass number and atomic mass is that mass number deals with individual atoms taken into consideration separately whereas atomic mass deals with the weighted average of the element including its isotopes. When considering elements in general, remember the atomic number never changes, but because there may be multiple isotopes, the mass number may change. Because in nuclear reactions mass number differs from atomic mass and the … Because in nuclear reactions mass number differs from atomic mass and the …
